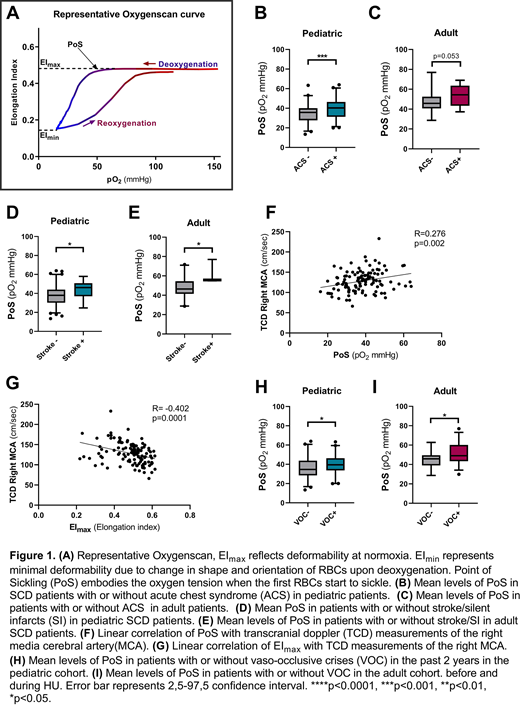
Background: In sickle cell disease (SCD), hemoglobin S (HbS) polymerizes upon deoxygenation, reducing red blood cell (RBC) deformability. RBC deformability can be measured over a gradient of oxygen tensions (pO2) with the Laser Optical Rotational Red Cell Analyzer (Lorrca) ektacytometer (RR Mechatronics). Oxygen gradient ektacytometry generates 3 key parameters: 1) EImax, RBC deformability at normoxia; 2) EImin, minimum RBC deformability upon deoxygenation; and 3) the point of sickling (PoS): the oxygen tension at which a 5% decrease in deformability is observed during deoxygenation, reflecting the patient-specific pO2 at which sickling begins (Figure 1A). Previously we showed that oxygen gradient ektacytometry-derived biomarkers correlate with measures of SCD disease severity and hemolytic rate (Rab et al. Blood 2018), and is associated with vaso-occlusive crisis (VOC) frequency (Rab et al, Blood 2019). In this study, we confirm these observations in 2 independent cohorts and extend it to occurrence of acute chest syndrome (ACS), stroke including silent cerebral infarction (SCI), and transcranial Doppler (TCD) outcome.
Methods: We analyzed 2 cohorts of SCD patients; an adult patient cohort of 53 SCD patients, enrolled at either University Medical Center Utrecht, The Netherlands (UMCU, n=25) or Hospital Lyon France (LIBM, n=28), and a pediatric patient cohort of 190 SCD patients enrolled at Texas Children's Hospital, USA (TCH). Subjects were HbSS or HbS/β-thalassemia, with a substantial number of subjects on hydroxyurea (HU) therapy (adult cohort 66% and pediatric cohort 86%), and not on chronic transfusion therapy. Correlations between oxygen gradient ektacytometry-derived biomarkers and the clinical complications of stroke or silent infarcts (SCI), ACS, VOC were assessed in both pediatric and adult patients. Patient groups generally did not significantly differ significantly by age, gender or HU treatment in the adult cohort except for age, which was lower in the ACS+ group (25.3years (y) compared to 32.0y) and also lower in the VOC+ group (27.1y compared to 35.8y). In the pediatric cohort, patient groups differed significantly in the ACS+ group compared to the ACS- group by age (ACS- group 8.37, ACS+ group 10.9y) and HU treatment (ACS- group 76%, ACS+ group 93%). Similarly, age was significantly higher in the Stroke+ group compared to the Stroke- group (14.0y compared to 9.3y), which was also found when studying VOC (VOC+ group 11.6y, VOC- group 8.2y).
Results: In the pediatric cohort, PoS was significantly higher in patients with ACS (mean 40.3 compared to 34.9 mmHg, p=0.0001, Figure 1B). In the adult cohort, PoS was also higher in those with ACS although this did not reach significance (p=0.053, Figure 1C).
In the pediatric cohort, PoS was higher in patients with stroke or SI (mean 43.0 mmHg compared to mean 37.3 mmHg, p<0.05), Figure 1D). This finding was replicated in the adult cohort (p<0.05, Figure 1E). EImin and EImax in both cohorts were significantly lower in patients who experienced stroke or SCI (all p<0.05). PoS was higher and EImin and EImax lower in subjects with elevated TCD measurements (all p<0.01, Figure 1F and G).
In the adult and pediatric patient cohort, PoS was higher in patients with recent VOC (both p<0.05, Figure 1H and I).
Differences in mean PoS between pediatric and adult patient cohorts could be due to differences in treatment, age, genetic background or technical differences between Lorrca devices. Lower significance levels found in the adult patient cohort could be due to smaller sample size.
Conclusion: We show that oxygen gradient ektacytometry provides functional, clinically relevant next generation biomarkers that are associated with ACS, stroke and VOC. This study therefore further validates the clinical usefulness of these biomarkers, in particular in relation to cerebral vasculopathy. Since our results merely describe an association and not causality further validation is warranted to establish how well oxygen gradient ektacytometry can assess disease severity. However, its parameters could already provide the clinician with information about patient RBC characteristics and sickling propensity that could aid in clinical decision making. Our results provide a rationale for further development of these biomarkers in the evaluation of novel therapies as part of clinical care, or clinical trial endpoints.
Rab:RR Mechatronics: Research Funding. Bos:RR Mechatronics: Research Funding. Cnossen:Takeda: Research Funding; Shire: Research Funding; Baxter: Research Funding; Bayer: Research Funding; Sobi: Research Funding; CSL behring: Research Funding; Nordic Pharma: Research Funding; Novo Nordisk: Research Funding; Pfizer: Research Funding. Wijk:Agios Pharmaceuticals Inc.: Research Funding; RR mechatronics: Research Funding. Sheehan:Emmaus: Research Funding; Global Blood Therapeutics: Research Funding; Novartis: Research Funding. van Beers:Pfizer: Research Funding; RR mechatronics: Research Funding; Agios: Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal